
| Homepage | |
| Group Members | |
| Publications | |
| 2002 - | |
| 1988 - 2001 | |
| Theses and | |
| Reports | |
| Picture Gallery | |
| Data | |
| Databases | |
| Software | |
| Job Vacancies | |
| Conferences, | |
| Workshops | |
| and Courses | |
| Local Info | |
| Group Info | |
| Projects | |
Salt-link tetrad involving Asp 251.
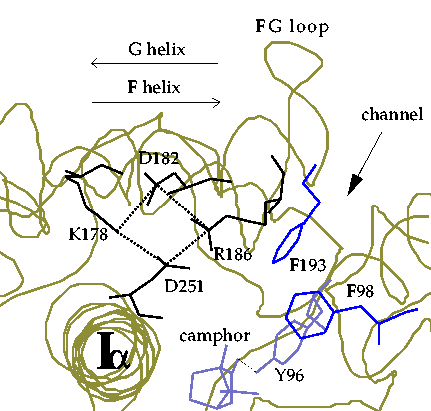
Figure 4. Schematic diagram of the region of the crystal structure of cytochrome P450cam around the salt-link tetrad involving Asp 251.
The tetrad of salt-links, formed by Lys 178, Asp 182, Arg 186 and Asp 251 provides a network of electrostatic interactions linking the F helix, G helix, and F-G loop to the I-helix adjacent to the heme cofactor. Electrostatics calculations of the stability of all the salt-links in cytochrome P450cam show the two salt-links to Asp 251 to be unusually stable indicating that the enzyme has evolved these salt-links to regulate substrate access.
Click here to go back!