
| Homepage | |
| Group Members | |
| Publications | |
| 2002 - | |
| 1988 - 2001 | |
| Theses and | |
| Reports | |
| Picture Gallery | |
| Data | |
| Databases | |
| Software | |
| Job Vacancies | |
| Conferences, | |
| Workshops | |
| and Courses | |
| Local Info | |
| Group Info | |
| Projects | |
Salt-link stability in cytochrome P450cam
The strengths of all salt-links relative to mutation to their hydrophobic isosteres were computed by numerical solution of the finite-difference Poisson-Boltzmann equation (Lounnas & Wade, in preparation). A continuum model of the solvent was used and the protein was treated as a continuous dielectric medium with partial atomic charges embedded in it. The distribution of the computed salt-link electrostatic free energies is shown in the histogram below. The computed free energy of each salt-link is given relative to its apolar uncharged isostere (hydrophobic isostere). Thus negative values indicate salt-links which are more stable than their hydrophobic isosteres. Of the four most stable salt-links, two are made to the heme propionate groups and two are to Asp251. Thus, it appears that cytochrome P450cam has evolved stable salt-links to perform important functional roles: keeping the heme cofactor in place and regulating substrate access to the active site.
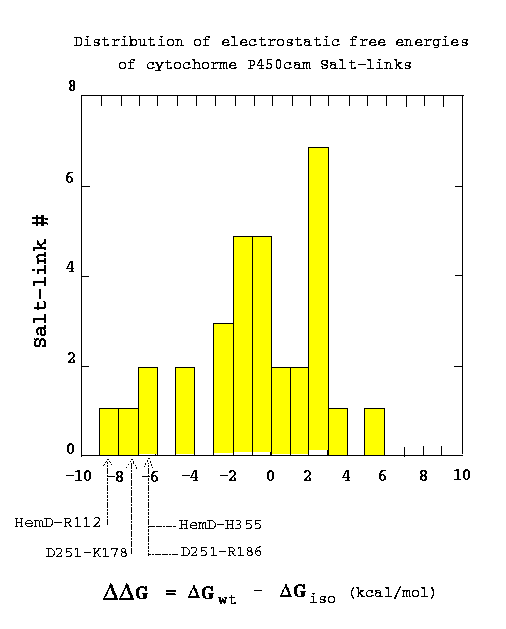
Figure 5. Distribution of the computed electrostatic free energies of the salt-links in cytochrome P450cam
Click here to go back!