
| Homepage | |
| Group Members | |
| Publications | |
| 2002 - | |
| 1988 - 2001 | |
| Theses and | |
| Reports | |
| Picture Gallery | |
| Data | |
| Databases | |
| Software | |
| Job Vacancies | |
| Conferences, | |
| Workshops | |
| and Courses | |
| Local Info | |
| Group Info | |
| Projects | |
Ionic strength and solvent dielectric dependence of K1d

Figure 2.
Effect of ionic strength on the first step equilibrium constant, K1d, for binding of camphor to wild-type cytochrome P450cam (open circles) and the D251N mutant (closed circles). Experiments were performed at 20 deg. C. in 100mM Tris-HCl at pH 7. [2].
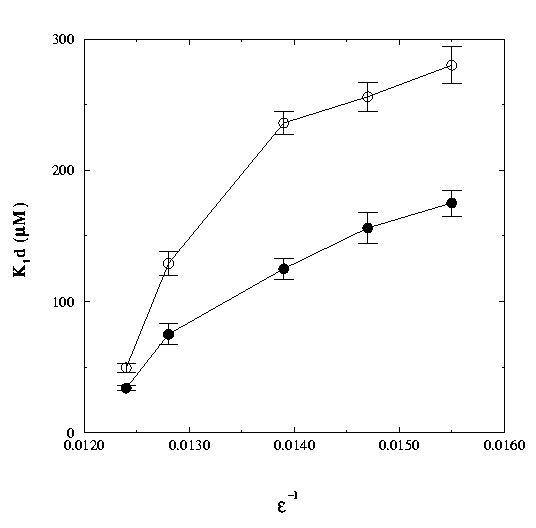
Figure 3.
Effect of altering the solvent dielectric constant on the first step equilibrium constant, K1d, for the binding of camphor to wild-type cytochrome P450cam (open circles) and the D251N mutant (closed circles). Experiments were performed at 20 deg. C. in a water/ethylene glycol mixture at pH 7. Addition of increasing amounts of salt to a 50% ethylene glycol-containing sample showed that the effect on K1d induced by the dielectric constant could be reversed by changes in the ionic strength [2].
Click here to go back!