Importance of the S1sub
pocket
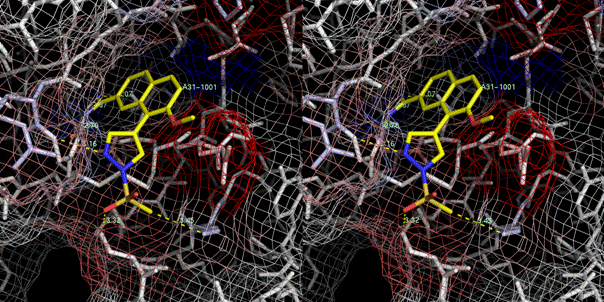
The S1sub pocket of urokinase is formed by the conserved Cys191-Cys220 disulfide bridge and the surrounding residues Gly219, Ser146, Gln192 and Lys143.
urokinase (A, PLS coefficients, no variable selection)
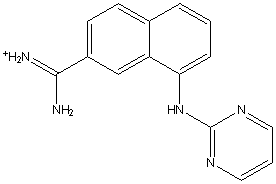
A31 (Ki=0.63 M) 1sqt (Nienaber, et al., 2000; Wendt, et al., 2004)
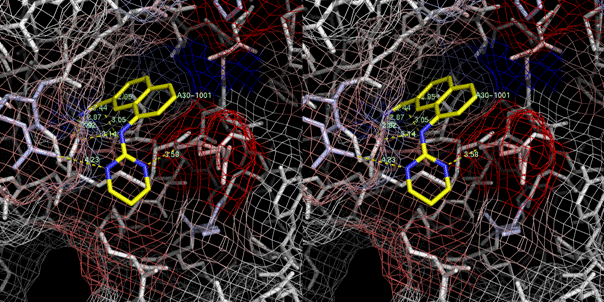
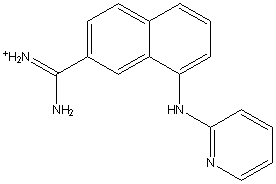
A30 (Ki=0.035 M) 1sqo (Nienaber,
et al., 2000; Wendt, et al., 2004)
Nienaber, V.L.,
Davidson, D., Edalji, R., Giranda,
V.L., Klinghofer, V., Henkin,
J., Magdalinos, P., Mantei,
R., Merrick, S., Severin, J.M., Smith, R.A., Stewart,
K., Walter, K., Wang, J., Wendt, M., Weitzberg, M.,
Zhao, X. and Rockway, T. (2000) Structure-directed
discovery of potent non-peptidic inhibitors of human
urokinase that access a novel binding subsite, Structure Fold Des, 8, 553-563.
Wendt, M.D., Geyer, A., McClellan, W.J., Rockway,
T.W., Weitzberg, M., Zhao, X., Mantei,
R., Stewart, K., Nienaber, V., Klinghofer,
V. and Giranda, V.L. (2004) Interaction with the S1
beta-pocket of urokinase: 8-heterocycle substituted and 6,8-disubstituted
2-naphthamidine urokinase inhibitors, Bioorg Med Chem Lett, 14, 3063-3068.
PLS coefficients for electrostatic interaction:
Gly216 ~0
Gln192 ~ +0.07
Arg217 -0.1
 A30 (Ki=0.035 M) 1sqo
A30 (Ki=0.035 M) 1sqo
H bond: NH--Gly216-0
favoured electrostatic interactions: NGln192-N
favoured repulsive
(neg. PLS coeff) electrostatic interaction (the
disfavoured interaction is less strong): N(=-0.20)Arg217-NH2
(0.9 kcal/mol uelec * -0.1 PLS coeff
= -0.09 kcal/mol)
 Ki=0.17
M
Ki=0.17
M
H bond: NH--Gly216-0
either no NGln192-N or no NArg217-NH2
interaction
 Ki=0.55 M
Ki=0.55 M
no H bond: NH--Gly216-0
either no NGln192-N or no NArg217-NH2
interaction
Results:
A qualitative comparison supports the retrieved
COMBINE model for the S1sub pocket.